UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): |
(Exact name of Registrant as Specified in Its Charter)
(State or Other Jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
|
||||
|
||||
(Address of Principal Executive Offices) |
|
(Zip Code) |
||
Registrant’s Telephone Number, Including Area Code: |
|
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
Trading |
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 8.01 Other Events.
ProKidney Corp. (the “Company”) has updated its investor presentation (the “Presentation”), which its senior management intends to use from time to time when interacting with investors and analysts, among others. The Presentation is available on the Company’s website at https://investors.prokidney.com/news-events/events-and-presentations. The Presentation is also attached hereto as Exhibit 99.1.
Item 9.01 Financial Statements and Exhibits.
|
(d) |
Exhibits. |
The exhibits filed as part of this Current Report on Form 8-K are listed in the index to exhibits immediately preceding the signature page to this Current Report on Form 8-K, which index to exhibits is incorporated herein by reference.
Exhibit No. |
|
Description of Exhibit |
99.1 |
|
104 |
|
Cover Page Interactive Data File (embedded within Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
PROKIDNEY CORP. |
|
|
|
|
Date: |
March 6, 2023 |
By: |
/s/ Todd Girolamo |
|
|
|
Todd Girolamo |

ProKidney Corporate Overview March 2023 Exhibit 99.1 A step closer to potential Dialysis Prevention React®[Renal Autologous Cell Therapy] Corporate Overview March 2023 Exhibit 99.1

Forward-looking Statements This presentation includes “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. ProKidney’s actual results may differ from its expectations, estimates and projections and consequently, you should not rely on these forward-looking statements as predictions of future events. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes,” “predicts,” “potential,” “continue,” and similar expressions (or the negative versions of such words or expressions) are intended to identify such forward-looking statements. These forward-looking statements include, without limitation, the Company’s expectations with respect to financial results, future performance, development and commercialization of products, if approved, the potential benefits and impact of the Company’s products, if approved, potential regulatory approvals, and the size and potential growth of current or future markets for the Company’s products, if approved. Most of these factors are outside of the Company’s control and are difficult to predict. Factors that may cause such differences include, but are not limited to: the inability to maintain the listing of the Company’s Class A ordinary shares on the Nasdaq; the inability to implement business plans, forecasts, and other expectations or identify and realize additional opportunities, which may be affected by, among other things, competition and the ability of the Company to grow and manage growth profitably and retain its key employees; the risk of downturns and a changing regulatory landscape in the highly competitive biotechnology industry; the inability of the Company to raise financing in the future; the inability of the Company to obtain and maintain regulatory clearance or approval for its products, and any related restrictions and limitations of any cleared or approved product; the inability of the Company to identify, in-license or acquire additional technology; the inability of Company to compete with other companies currently marketing or engaged in the biologics market and in the area of treatment of kidney diseases; the size and growth potential of the markets for the Company’s products, if approved, and its ability to serve those markets, either alone or in partnership with others; the Company’s estimates regarding expenses, future revenue, capital requirements and needs for additional financing; the Company’s financial performance; the Company’s intellectual property rights; uncertainties inherent in cell therapy research and development, including the actual time it takes to initiate and complete clinical studies and the timing and content of decisions made by regulatory authorities; the impact of COVID-19 or geo-political conflict such as the war in Ukraine on the Company’s business; and other risks and uncertainties indicated from time to time in the Company’s filings with the Securities and Exchange Commission. The Company cautions readers that the foregoing list of factors is not exclusive and cautions readers not to place undue reliance upon any forward-looking statements, which speak only as of the date made. The Company does not undertake or accept any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements to reflect any change in its expectations or any change in events, conditions or circumstances on which any such statement is based.

The Problem The Goal The Product The Plan Contribution to Society REACT® is a proprietary cell therapy using the patient’s own kidney cells Preclinical activity and mechanism of action translated to clinical activity REACT® includes three specific cell types with the potential to help restore kidney function Phase 3 clinical program received FDA and EMA guidance and RMAT designation; proact 1 underway Ongoing Phase 2 trials to expand clinical insights Target commercial launch YE 2026 $130 billion Medicare cost to care for the 40 million CKD/ESKD patients in U.S. 75 million CKD patients in the U.S. and EU Currently, no treatment options (other than transplant) exist to stop decline of kidney function Afflicted population includes millions of moderate-severe diabetic CKD patients Potential label expansion could expand TAM to nearly all chronic kidney diseases Reduce overall cost to the healthcare system Stop kidney failure Reduce the lifetime cost of care for CKD patients and payers What is ProKidney? REACT® aims to be the world leader in treating Chronic Kidney Disease (CKD)

What is the Problem? CKD is Serious Public Health Problem Today $80B+ Private insurance may pay up to 4x Medicare costs Medicare spend on Chronic Kidney Disease $50B+ Medicare spend on End Stage Renal Disease $93K+ Medicare annual cost per patient for dialysis One of the largest healthcare expenditure categories in the U.S. CKD Population Highly prevalent in the U.S. and EU 50% CAGR EU US 82M 61M 74M 91M Kidney failure costs represent one of the largest line items of Medicare Budget

Source: The New England Journal of Medicine. EvaluatePharma Note: 2026 sales estimates for therapies reflect all indications and are not limited to CKD While New Therapies Are a Step Forward, Patients Still Lose Kidney Function Recently approved CKD drugs incrementally slow eGFR loss, but CKD has no known cure Estimated global market sales of Canagliflozin, Dapagliflozin and Empagliflozin are ~$10B in 2026 REACT® evaluating more severe CKD (20-50 eGFR; mean <30 ml/min/1.73m2 in Ph 2) and data suggests potential to preserve kidney function in patients with very high risk of kidney failure +2.2 ml/min/1.73 m2 WW Sales ~$740mm (2026) +1.1 ml/min/1.73 m2 WW Sales ~$3.7bn (2026) +1 ml/min/1.73 m2 WW Sales ~$5.1bn (2026) Current standard of care for DKD Stage 2/3a (eGFR above 40) merely slows the eventual loss of kidney function Standard of Care has Limitations Current Therapies are Blockbusters While patients continue to lose kidney function on existing therapies, those therapies still generate nearly $10 billion WW sales annually Dapagliflozin: +1.1 ml/min/1.73 m2 Improvement Baseline mean eGFR of 43 ml/min/1.73 m2 Canagliflozin: +2.2 ml/min/1.73 m2 Improvement Baseline mean eGFR of 56 ml/min/1.73 m2 Empagliflozin: +1 ml/min/1.73 m2 Improvement Baseline adjusted mean eGFR of 76 ml/min/1.73 m2

Total addressable market data for the year ended 2020 is based on certain estimates of management. This information may prove to be inaccurate because of the method by which the underlying data for the estimates was obtained or because this information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties REACT’s Addressable U.S. Patient Population Initially targeting advanced type 2 diabetic CKD with potential for multiple label expansion indications 2020 U.S. Patients (mm)1 Total CKD CKD Stage 3&4 U.S. Prevalence of CKD 38.5 17.6 CKD Stage 3&4 Caused by Diabetes U.S. CKD Stage 3&4 Caused by Diabetes with 20-50 eGFR 8.3 4.4 (15% of U.S. Adult Population)

REACT®: REnal Autologous Cell Therapy for CKD Advancing a comprehensive clinical plan to demonstrate commercial potential REACT® Phase 3 DKD Trials proact 1: Ongoing enrollment in U.S., Canada, and EU; Interim anticipated YE24 proact 2: 1H23 ROW enrollment; Interim anticipated by YE25 Global Phase 3 blinded, sham-controlled trials to establish safety & efficacy of REACT® Stage 3b / 4 DKD (eGFR ≤ 50 – 20) FDA-defined time-to-event endpoints REGEN-007 Phase 2 Enrollment ongoing Interim results anticipated 2H23 Safety & efficacy Open-label trial DKD Stage 3 / 4 (eGFR ≤ 50 – 20) Bi-lateral kidney and dose triggers Cryopreserved commercial formulation Ph3 “preview” REGEN-003 Phase 2 Trial completed Results published 1Q23 Safety & efficacy of REACT® DKD Stage 4 / 5 (eGFR < 20 – 14) Identify potential re-dosing triggers Assess impact on progression and time to dialysis in patients with imminent risk of renal failure/dialysis 2024 and beyond 2H 2023 1H 2023 $506M cash sufficient to fund these key milestones, and to interim Phase 3 data FDA / EMA agreement on pivotal study design RMAT designation in U.S. Potency Assay Matrix alignment Cash Position (as of 9/30/22) Regulatory REGEN-015 Multi-dose trial Launch projected Mid-2023 Safety & efficacy of repeat dosing Previously treated DKD Repeat dosing and durability
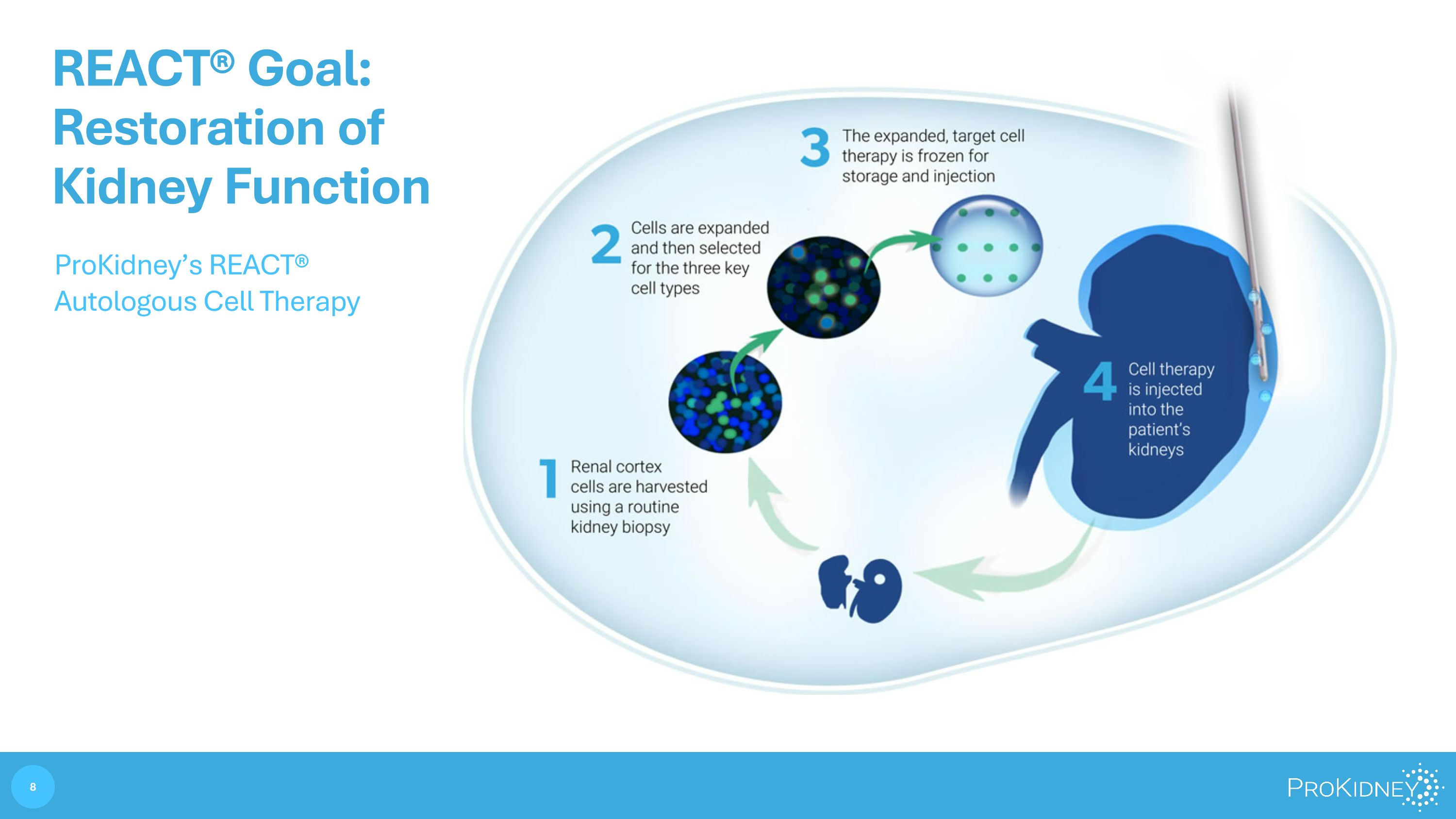
REACT® Goal: Restoration of Kidney Function ProKidney’s REACT® Autologous Cell Therapy REACT® Goal: Restoration of Kidney Function ProKidney’s REACT® Autologous Cell Therapy 1 Rental cotex cells are harvested using a routine kidney biopsy 2 Cells are expanded and then selected for the three key cells types 3 The expanded, target cell therapy is frozen for storage and injection 8 ProKidney

Kelly et al, Am J Physiol Renal Physiol 299: F1026-F1039, 2010. Bruce et al, Regenerative Medicine 10(7), 2015 Preclinical studies in rodents and canines, data on file. Remodeling and Renovation of Nephrons REACT® aims to preserve kidney function for dialysis-free living 26 3 Types of Cells in Adult Kidney Types of Progenitors in REACT® REACT®: Autologous Homologous Triple Cell admixture Renal cells Cap Mesenchyme, Podocytes, and Ureteric Bud: Six-2/OSR1/PAX-2 (Cap Mesenchyme) RET (Ureteric Bud) Podocin / Nephrin Intra-tubular and Glomerular (REACT® – Blue) Interstitial (REACT® – Blue) Cells shown to distribute throughout kidney and integrate into nephrons and interstitium 150 x 106 REACT® @1.5mL 50 x 106 REACT® @ 0.5mLs 25 X106REACT® @ 0.25mLs

REACT® MoA in CKD – ASN November 2022 Nephrogenic potential of SRCs may underlie renal restorative and reparative activity observed to-date SRCs participate in processes associated with kidney development SRCs forms organoids, which self-assemble into tubules in vitro SRC/REACT® in human cell culture SRC/REACT® preserves kidney microarchitecture REACT® treated kidney shows reduced glomerular and interstitial fibrosis and inflammation, protein leakage and tubular ectasia Control/Normal DKD + REACT® DKD

Kelly et al, Am J Physiol Renal Physiol 299: F1026-F1039, 2010. Bruce et al, Regenerative Medicine 10(7), 2015 Preclinical studies in rodents and canines, data on file. REACT® Impact on Kidney Function Preclinical data suggest REACT® treatment may improve kidney function via multiple mechanisms REACT® Impact on Kidney Function Preclinical data suggest REACT® treatment may improve kidney function via multiple mechanisms CONTROLS INFLAMMATION Restores renal Function VEGF promotes angiogenesis to form new capillaries REACT insinuates with mesangial cells REACT RESTORES Glomerular Cells REACT replaces podocytes INTEGRATES REACT REPLACES Damaged Nephron Cells REVERSES FIBROSIS Fibroblasts become inactivate, and basement membrane remodeling takes place Cell-cycle restarts and replaces tubular epithelial cells REACT REPLACES Tubular Cells Kelly et al, Am J Physiol Renal Physiol 299: F1026-F1039, 2010. Bruce et al, Regenerative Medicine 10(7), 2015 Preclinical studies in rodents and canines, data on file.11 ProKidney

REACT® Trials Designed to Address Multiple Areas of CKD Potential therapeutic indications Lead Platform Programs (Clinical Development) PRECLINICAL IND PHASE 1 PHASE 2 PHASE 3 STATUS REACT® Diabetic Kidney Disease (DKD) Diabetes Type II – Prevent/Delay CKD 3/4 (20-50 ml/min/1.73m2, N = 81) Diabetes Type II – Prevent/Delay CKD 3/4 (20-50 ml/min/1.73m2, N = 1,200) Diabetes Type II – Delay CKD 4/5 (14-20 ml/min/1.73m2, N = 10) Diabetes Repeat Dose Prevent/Delay CKD 3/4 (20-50 ml/min/1.73m2, N= 50*) Multi / extended-dosing for previously REACT-treated patients Long term follow-up study for patients previously treated with REACT REACT® Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) Congenital Anomalies – Prevent/Delay (14-50 ml/min/1.73m2, N= 5) Fully Enrolled Trial Completed Enrolling Ongoing US/ OUS 1H2023 Enrollment 2Q2023 007 Phase 2 (006/016) Phase 3 015 Phase 1/2 Phase 1 004 003 Phase 2 002 Trial Completed Phase 2 008 Phase 3 Enrollment 3Q2023 REACT® Trials Designed to Address Multiple Areas of CKD Potential therapeutic indications Lead Platform Programs (Clinical Development) REACT® Diabetic Kidney Disease (DKD) REACT® Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) Diabetes Type II – Prevent/Delay CKD 3/4 (20-50 ml/min/1.73m2, N = 81) Diabetes Type II – Prevent/Delay CKD 3/4 (20-50 ml/min/1.73m2, N = 1,200) Diabetes Type II – Delay CKD 4/5 (14-20 ml/min/1.73m2, N = 10) Diabetes Repeat Dose Prevent/Delay CKD 3/4 (20-50 ml/min/1.73m2, N= 50*) Multi / extended-dosing for previously REACT-treated patients Long term follow-up study for patients previously treated with REACT Congenital Anomalies – Prevent/Delay (14-50 ml/min/1.73m2, N= 5) PRECLINICAL IND PHASE 1 PHASE 2 PHASE 3 STATUS Phase 2 002 Phase 3 proact proact Phase (006/016) 2 003 Phase 2 007 Phase 1/2 015 Phase 3 008 Phase 1 004 Fully Enrolled Ongoing US/ OUS 1H2023 Trial Completed Enrolling Enrollment 2Q2023 Enrollment 3Q2023 Trial Completed Egfr 50-20 ml/min/1.73m2 Unilateral injections Frozen product eGFR 20-140 ml/min/1.73m2 Bilateral injection Re-dosing ProKidney 12

Building a Comprehensive Data Package Clinical program designed to expand potential patient population and support premium pricing Assess potential benefit of multiple REACT® doses (REGEN-015) Determine durability of REACT® injection (REGEN-008 and RMCL-002) Identify re-dosing triggers (REGEN-007) Assess potential benefit of bilateral REACT® injections (REGEN-007) Support potential label expansion (REGEN-003) Support durability, reimbursement and value-based pricing (REGEN-008) 006 1st Registrational 016 2nd Registrational 015 Repeat dosing & Product Lifecycle 003 Label Expansion 007 re-dose triggers & preview of Phase 3 REACT® Potential approval for DKD, >8M eligible patients U.S. and EU Goal: Prevent the progression to dialysis 008 Durability Clinical Trial Package Supporting Registration 002 Basis for Phase 3 Building a Comprehensive Data Package Clinical program designed to expand potential patient population and support premium pricing Assess potential benefit of multiple REACT® doses(REGEN-015) Determine durability of REACT® injection (REGEN-008 and RMCL-002)Identify re-dosing triggers (REGEN-007) Assess potential benefit of bilateral REACT® injections (REGEN-007) Support potential label expansion (REGEN-003) Support durability, reimbursement and value-based pricing (REGEN-008) REACT® Potential approval for DKD, >8M eligible patients U.S. and EU Goal: Prevent the progression to dialysis Clinical Trial Package Supporting Registration 008 Durability proact 006 1st Registrational proact 016 2nd Registrational 007 re-dose triggers & preview of Phase 3 015 Repeat dosing & Product Lifecycle 002 Basis for Phase 3 003 Label Expansion 13 ProKidney 13

Key Entry Criteria Type 2 Diabetic Mellitus (DKD) Male or female 30-80 years of age eGFR ≥20 and ≤50 mL/min/1.73m2 Not on renal dialysis, HbA1c <10% Day –60 to Day 0 Screening Visit and Biopsy Deferred n= 42 R 1:1 1st REACT® Injection 2nd REACT® Injection EOS Month 24 1st REACT® Injection 2nd REACT® Injection EOS Month 24 Active n = 41* Follow-up visits conducted at 3-month intervals after 3 months post 2nd REACT® injection for 24 months until End of Study part 1 * 2 ‘Active’ patients dropped out of the study resulting in n=39 in subsequent slides. RMCL-002: Study in Diabetics with CKD Stages 3A, 3B & 4 Clinical trial design = 6 months

REACT ® Stabilizes Kidney Function Baseline eGFR (Cystatin-C): 38.4 active vs 37.3 for deferred patients + 1.1 points higher Baseline eGFR (Creatinine): 34.5 active vs 32.2 for deferred patients + 2.3 points higher REACT® Renal function improved by an absolute improvement over 24 months of + 4.6 ml/min/1.73m2 Standard of Care Progressive decline in kidney function over 12 months of ‒3.6 ml/min/1.73m2 A characteristic of SOC for CKD 3a, 3b, and 4 Note: As of March 1, 2022 RMCL-002: Preliminary Results from Study in Diabetics with CKD Stages 3A, 3B & 4 Comparing effect of REACT® vs. standard of care (SoC) in Phase 2 study RMCL -002: Preliminary Results from Study in Diabetics with CKD Stages 3A, 3B & 4 Comparing Effect of REACT vs . Standard of care (Soc)in Phase 2 study REACT Renal function improved by an absolute improvement over 24 months of +4.6 m1/min/1.7m2 Standard of Care Progressive decline in kidney function over 12 months of ‒3.6 ml/min/1.73m2 A characteristic of SOC for CKD 3a, 3b, and 4 Active patients (N=39) Effect after 1st injection vs Deferred Patients on SOC (N=40) Screening 1st Inj 1stInj+ 3m Follow-up 2nd Inj 2nd+ 3m Follow-up 2nd Inj + 6m Follow-up 2nd Inj + 9m Follow- up 2nd Inj + Follow-up 2nd Inj + 15m Follow up 2nd Inj +18m Follow up Screening Biopsy Obs 3m Obs 6m Obs 9m Obs 12m eGFR(mL/min/1.73m2)40 35 30 25 20 -6.0 -3.0 0.0 3.0 6.0 9.0 12.0 15.0 18.0 21.0 24.0 33.9 31.7 32.8 32.0 28.4 37.4 Active Cohort Deferred Cohort Months Note As of March 1,2022 15 Prokinney

REGEN-003: Phase 2 Study in Stage 4/5 Diabetic CKD Patients Clinical trial design: Participants' baseline rate of eGFR decline served as comparator UACR mean 3190 mg/gr; mean eGFR 15.5 mL/min/1.73m2; >90% probability of dialysis initiation No other marketed drug is indicated for these patients = 6 months Key Entry Criteria Type 2 Diabetic Mellitus (DKD) Male or female 30-65 years of age eGFR ≥14 - ≤20 mL/min/1.73m2 Not on renal dialysis, HbA1c <10% Follow-up visits conducted at 3-month intervals after 3 months post 2nd REACT® injection for 24 months until End of Study REGEN-003: Phase 2 Study in Stage 4/5 Diabetic CKD Patients Clinical trial design: Participants' baseline rate of eGFR decline served as comparator UACR mean 3190 mg/gr; mean eGFR 15.5 mL/min/1.73m2; >90% probability of dialysis initiation No other marketed drug is indicated for these patients n=10 1st REACT Biopsy Injection 2nd REACT Injection EOS month24 = 6 months Key Entry Criteria Type 2 Diabetic Mellitus (DKD) Male or female 30-65 years of age eGFR ≥14 - ≤20 mL/min/1.73m2 Not on renal dialysis, HbA1c <10% Follow-up visits conducted at 3-month intervals after 3 months post 2nd REACT® injection for 24 months until End of Study 18 ProKidney

REGEN-003: Pre-Dialysis Patients Benefit from REACT 7/10 patients had 30% increase in dialysis free living (median of ~16 mo.) 6/10 patients observed to have improved eGFR or stabilization 2/10 patients have preservation of renal function >2+ years post injection Mean extrapolated REACT Treated LABEL EXTENSION Stavas, et al. Renal Autologous Cell Therapy in Type 2 Diabetes with Late Stage 4 Diabetes-Related Chronic Kidney Disease: Trial Design and Early Analysis. J Blood Purification. Published Online Jan 2023 LABEL EXTENSION REGEN-003: Pre-Dialysis Patients Benefit from REACT 7/10 patients had 30% increase in dialysis free living (median of ~16 mo.) 6/10 patients observed to have improved eGFR or stabilization 2/10 patients have preservation of renal function >2+ years post injection all 003 Patients (N= 10): Average Change in eGFR Screening Biopsy 1st Inj 1st + 3m Follow up 2nd Inj 2nd Inj 3m Follow Up 2nd Inj + 6m Follow Up 2nd Inj +9m Follow 2nd Inj + 12m Follow up 2nd Inj +15m Follow 2nd Inj +21m Follow Up 2nd Inj + 24m Follow 25 20 15 10 eGFR (Ml/Min/1.73m2)17.5 15.5 -0.7 17 N= 10 10 10 6 9 8 7 6 3 3 3 2 2 -6 -3 0 3 6 9 12 15 18 21 24 27 30 Months CKD- EPI Creatinine Equation (2009) 20 15 10 5 0 -10 0 10 20 30 REACT Treated Mean Extrapolated Months since First REACT Injection Stavas, et al. Renal Autologous Cell Therapy in Type 2 Diabetes with Late Stage 4 Diabetes-Related Chronic Kidney Disease: Trial Design and Early Analysis. J Blood Purification. Published Online Jan 2023 ProKindey

Consistently aiming to mitigate procedure-related risks while preserving kidney function for late-stage CKD patients Interim Safety Profile: Safety of REACT in Phase 2 Diabetic CKD Stages 3A, 3B, 4, & 5 and CAKUT Serious Adverse Event n Hematoma* 1 Transfusion* 1 Acute Kidney Injury* 1 Macroscopic Hematuria 0 Angiographic Intervention 0 Surgery 0 Death 0 CKD progression 1 Renal vascular event 1 Cortical Scar 1 Renal arteriovenous fistula 0 Events observed in 4/83 participants. *Hematoma, transfusion, & AKI events occurred in one patient pre-needle design-change in Sept. 2017, other SAE events occurred post-needle design change. Data as 2/23. Source: Stavas et al. SIR March 2023. -003 Procedure-related events: Renal Related (N=10 pt biopsies, 19 injections) Serious Adverse Event n Hematoma 2 Transfusion 0 Acute Kidney Injury 2 Macroscopic Hematuria 0 Angiographic Intervention 0 Surgery 0 Death 0 CKD progression 1 Renal vascular event 0 Cortical Scar 0 Renal arteriovenous fistula 1 -002 Interim procedure-related events: Renal Related (N=83 pt biopsies, 132 injections) Events observed in 3/10 participants. No cell product related SAEs were reported. Source: Stavas et al. Blood Purif 2023;52:114–121 DOI: 10.1159/000527582 -004 Procedure-related events: Renal Related (N=5 pt biopsies, 9 injections) Serious Adverse Event n Hematoma 0 Transfusion 0 Acute Kidney Injury 0 Macroscopic Hematuria 0 Angiographic Intervention 0 Surgery 0 Death 0 CKD progression 0 Renal vascular event 0 Cortical Scar 0 Renal arteriovenous fistula 0 Events observed in 4/39 participants. *One hematoma associated with an injection. Two hematomas, two AKI, and one hematuria occurred following biopsy. Data on file and as of 1/23. Serious Adverse Event n Hematoma* 4 Transfusion 1 Acute Kidney Injury 2 Macroscopic Hematuria 1 Angiographic Intervention 0 Surgery 0 Death 0 CKD progression 0 Renal vascular event 0 Cortical Scar 0 Renal arteriovenous fistula 0 -007 Interim procedure-related events: Renal Related (N=39 pt biopsies, 42 injections) Events observed in 0/5 participants. No cell product related SAEs were reported. Data on file and as of 1/23. 202 REACT® injections administered to date in Phase 1 and 2 clinical studies REACT has been tolerated by patients with moderate-severe CKD at high risk for renal failure

REACT Procedure Continued to Demonstrate a Complication Rate Below a Standard Kidney Biopsy REACT procedure in Phase 2 clinical trials was tolerated with a safety profile similar to a standard biopsy Category Biopsy # of patients (%) (N=133) REACT Injection # of patients (%) (N=202) Hematoma 4 (3.0) 3 (1.5) Pain 0 3 (1.5) Hematuria 1 (0.7) 0 Transfusion 1 (0.7) 1 (0.5) Bleed + intervention 0 0 Death 0 0 REACT Phase 2 Safety Profile Summary Includes data available from ongoing and completed phase 2 trials. Data on file and as of 3/1/2023. REACT Procedure Continued to Demonstrate a Complication Rate Below a Standard Kidney Biopsy What are the complications associated with native kidney Biopsy CJASN Clinical Journal of the American Society of Nephrology Systematic review and meta – analysis of th literature Published from jan 1983 to mar 2018 1139 manuscripts in initial pubmed search Pre – determined selection Criteria 87 manuscripts in final analysis Complication rates of native kidney biospsies performrd using automated devices under kidney imaging native kidney biopsies n + 118,064 events 30-79 years patient age range 47% Female 11% 1.6 % 4.3% 0.3% 3.5% 0.06% 1in 1,1667 Mainbiopsy complications hematoma bleeding requiring Transfusions pain at biopsy site macroscopic hematuria Death in patients who undergo a native kidney biopsy Complication rates were higher in: Hospitalized patients Patuents with acute kidney injury Conclusions Although the native kidney biopsy is an invasive diagnostic procedure, the rates of bleeding Complications are low . Albeit rare, death can occur post biopsy. Complications are more frequenty seem after Hospitalization and acute Kidney injury Emillio D. Poggio, Robyn L. McClelland, Kristina blank, Spencer Hansen, et al. systematic Review and meta- analysis of native kidney Biopsy Complications. CJASN REACT Phase 2 Safety Profile Summary Category Biopsy # of patients (%) (N=133) REACT Injection # of patients (%) (N=202) Hematoma Pain Hematuria Transfusion Bleed + intervention Death 4 (3.0) 0 1(0.7) 1(0.7) 0 0 3(1.5)3(1.5)0 1(0.5)0 0 Includes data available from ongoing and completed phase 2 trials. Data on file and as of 3/1/2023. REACT procedure in Phase 2 clinical trials was tolerated with a safety profile similar to a standard biopsy 19 Prokidney

Time-to-Event Primary Composite Endpoint: At least 40% reduction in eGFR; eGFR<15mL/min/1.73m² sustained for 30 days and/or chronic dialysis, and/or renal transplant; or Death from renal or cardiovascular causes Day - 60 to Day 0 Screening Visit SHAM Cohort n=300 REACT Cohort n=300 R 1:1 Sham 1st REACT® Injection Sham 2nd REACT ® Injection Sham Biopsy End of Study (EOS) Global Trial End Date (GTED) 1st REACT® Injection 2nd REACT® Injection Biopsy End of Study (EOS) Global Trial End Date (GTED) = 6 months = 3 months REACT® Registrational Program: 1 & 2 (REGEN-006 / 016) First 1 patients enrolled in 2022 Key Entry Criteria Type 2 Diabetic Mellitus (DKD) Male or Female 30-80 years of age eGFR ≥20 and ≤50 mL/min/1.73m2 Not on renal dialysis, HbA1c <10% UACR 300 - 5,000 mg/g

* EMA: European Medicines Agency, RMAT: Regenerative Medicine Advanced Therapy, BLA: Biologics License Application, SGLT2i: Sodium-glucose Co-transporter 2 inhibitor, HTA: Health technology assessment, MHRA: Medicines & Healthcare products Regulatory Agency of the United Kingdom, NICE: National Institute for Health and Care Excellence in the United Kingdom REACT® Registrational Program Regulatory & reimbursement engagement plan: Diabetic Kidney Disease FDA / EMA* HTA Conducting ‘Gold Standard’ Two Adequate and Well Controlled RCT for BLA* Approval RMAT* designation provides potential for accelerated approval pathway in U.S. Time to event and composite endpoints align with registration study designs previously used by other FDA approved CKD therapies (i.e., SGLT2i) HTA* Potential Healthcare Savings Validate for HTA’s REACT’s® effect of delaying the time to ESRD (dialysis/transplant) as a potential major healthcare system cost savings MHRA/NICE* parallel advice for UK U.S., France, Germany HTAs

High Patient Acceptance for New Medications Survey participants were overwhelmingly willing to take a medication - even if side effects occurred (93.6%) Inker et al Am J Kidney Dis. 80(4):513-526 Panelist with the CKD stage 3a stated: “….if I did see an appreciable decrease in my kidney health then I’m sure I would be much more open to trying some things.” Panelist with CKD stage 3b stated: “Anything to help … slow [the] progress of the kidney disease — I’m all for it.” REACT ~75% High Patient Acceptance for New Medications Survey participants were overwhelmingly willing to take a medication – even if side effects occurred (93.6%) Current staging system for CKD and treatment considerations ACR Stages 1 <30 No CKD 2 30-300 3 >300 Nephrotic GFR Stages 1 >90 2 60-89 3a 45-59 3b 30-44 4 15-29 5 <15 REACT CKD stage/Risk no CKD Moderately high risk High risk Very high risk Goal for treatment Prevent development Prevent progression and complications Indication for treatment Current area of controversy Agreement that treatments are indicated Panelist with the CKD stage 3a stated: “ if I did see an appreciable decrease in my kidney health then I’m sure I would be much more open to trying some things.”” Panelist with CKD stage 3b stated: “Anything to help … slow [the] progress of the kidney disease- I’m all for it.” Patient responses to question asking about the like lihood of taking a new medication to prevent kidney failure: You have a 20% chance of developing kidney failure… over 20 years over 10 years over 5 years 0% 10% 20% 30% 40% 50% Very likely likely neutral somewhat likely not likely ~75% 22 Inker et al Am J Kidney Dis. 80(4):513-526 PROKIDNEY

ESRD Patients Remain on Dialysis for 5-10 Years on Average Source: United States Renal Data System - USRDS 2020 Annual Report (https://adr.usrds.org/2020/about-the-new-adr), National Kidney Foundation (https://www.kidney.org/atoz/content/dialysisinfo#how-long-can-you-live-dialysis), company estimates Significant Cost Savings Potential Potential impact of a disease-modifying product Improve patients’ quality of life Enable patients to be productive Reduce burden to families Reduce healthcare system costs A disease-modifying drug in CKD could reduce cost of kidney failure Estimated ESRD Cost Per Patient (Illustratively Based on 5 Years on Dialysis) Incremental Private Insurance Cost X 5 Years ~$100k Up to ~$400k ~$500k Up to ~$2M Medicare Cost / Year Private Insurance Cost / Year Cumulative 5-Year Cost / Patient

Staged construction of commercial scale manufacturing facilities In-house manufacturing supports clinical program Scalable to meet initial commercial demand upon regulatory approval Future facilities will be built to meet market demand Manufacturing toward clinical and commercial opportunities Phase 2 COGS ~$100K / patient Anticipate COGS to potentially decrease by approximately 50% through scale-up for commercialization Supply chain Automation Bioprocess developments Formulation Cost of Goods Sold (COGS) Manufacturing Strategies Infrastructure strategy to reduce COGS and expand addressable market Manufacturing Strategies Infrastructure strategy to reduce COGS and expand addressable market Manufacturing toward clinical and commercial opportunities Staged construction of commercial scale manufacturing facilities In-house manufacturing supports clinical program Scalable to meet initial commercial demand upon regulatory approval Future facilities will be built to meet market demand Cost of Goods Sold (COGS) Phase 2 COGS~$100K / patient Anticipate COGS to potentially decrease by approximately 50% through scale-up for commercialization Supply chain Automation Bioprocess developments Formulation Anticipate ~50% Reduction in COGS Labor Materials Other Phase 2 COGS $100k/pt COGS at commercial scale 24 PROKIDNEY

Dr. Joe Stavas SVP, Global Head Clinical Development Ashley Johns SVP, Global Head Clinical Operations Dr. Darin Weber Chief Regulatory Officer Dr. Tim Bertram Chief Executive Officer Dr. Deepak Jain Chief Operating Officer James Coulston Chief Financial Officer Pablo Legorreta Chairman of the Board José Ignacio Jiménez Santos William Doyle Jennifer Fox Dr. Tim Bertram Dr. Alan Lotvin Dr. John Maraganore Dr. Brian Pereira Dr. Uma Sinha World-class Leadership and Board of Directors Todd Girolamo Chief Legal Officer & Secretary Mary Weger Chief People Officer World-class Leadership and Board of Directors Dr. Tim Bertram Chief Executive Officer REGEMEDTX Nexlmmune inRegen Pfizer Dr. Deepak Jain Chief Operating Officer REGEMEDTX Baxter Merck James Coulston Chief Financial Officer TARGACEPT EY Todd Girolamo Chief Legal Officer & Secretary Caladrius BIOSCIENCES LEERINK Dr. Darin Weber Chief Regulatory Officer Medeor Therapeutics Biologics CONSULTING mesoblast the adult stem cell company FDA Mary Weger Chief People Officer Celgene Aegerion Pharmaceuticals Therachon achieving potential Dr. Joe Stavas SVP, Global Head Clinical Development UNIVERSITAS CREIGHTONIANA 1878 Duke UNIVERSITY UNC SCHOOL OF MEDICINE Ashley Johns SVP, Global Head Clinical Operations REGEMED TX tengion Regenerative medicine brought to life, PMGResearch Pablo Legorreta Chairman of the Board ROYALTY PHARMA SCIENTIA PRO BONO HUMAN GENERIS The Rockefeller University 1901 BROWN UNIVERSITY The New York Academy of Sciences OPEN MEDICAL INSTITUTE Medical Education Beyond Borders Kidney health Foundation HSS LAZARD FRERES GESTION Dr. Tim Bertram William Doyle novocure Jennifer Fox Nuvation Bio Dr. Alan Lotvin CVS Health Dr. John Maraganore Alnylam PHARMACEUTICALS Dr. Brian Pereira Visterra Bridgebio Dr.Uma Sinha Jose Ignacio Jimenez Santos INBURSA Afore 25 PROKIDNEY
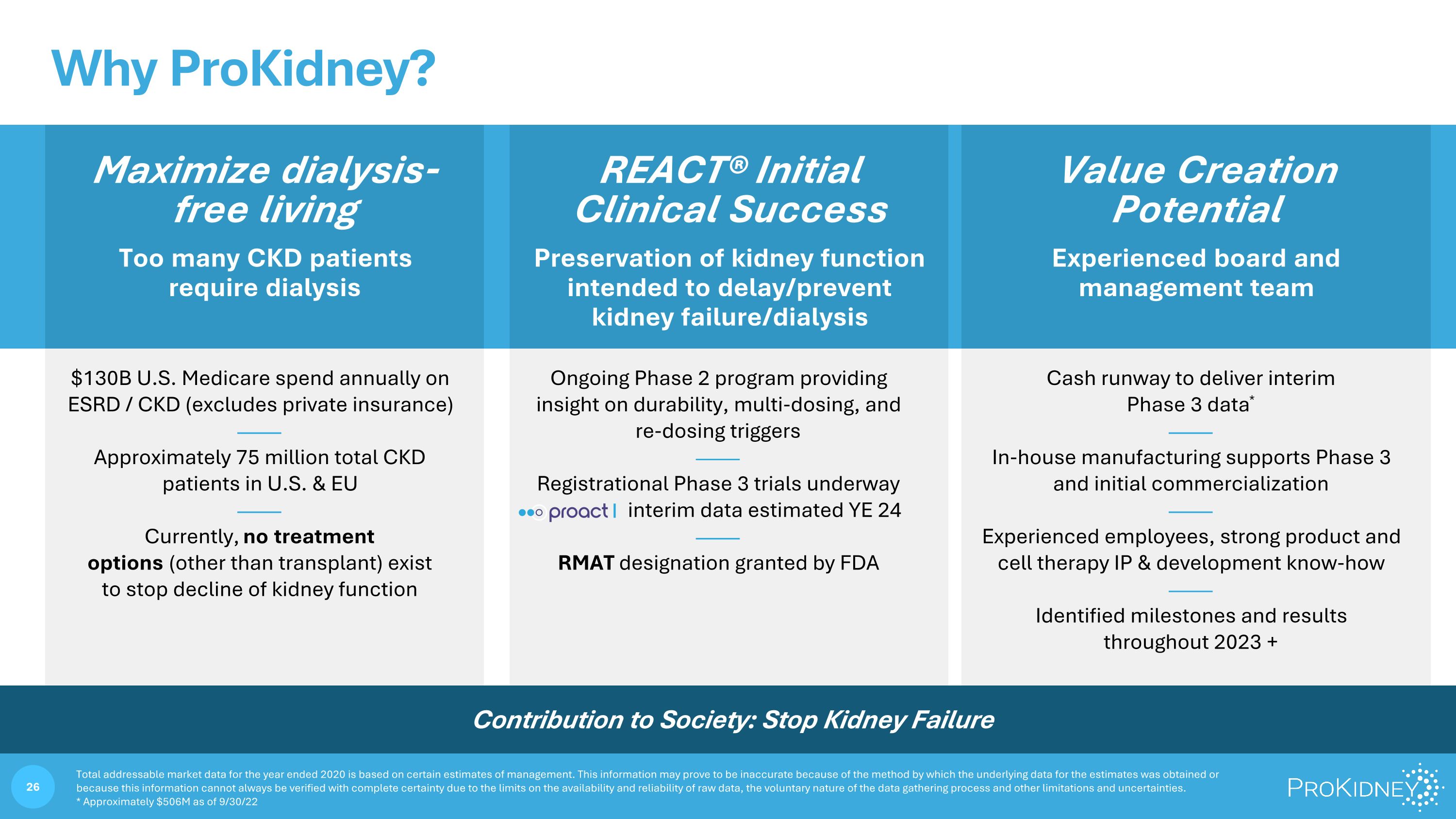
REACT® Initial Clinical Success Maximize dialysis-free living Too many CKD patients require dialysis Preservation of kidney function intended to delay/prevent kidney failure/dialysis Experienced board and management team $130B U.S. Medicare spend annually on ESRD / CKD (excludes private insurance) —— Approximately 75 million total CKD patients in U.S. & EU —— Currently, no treatment options (other than transplant) exist to stop decline of kidney function Ongoing Phase 2 program providing insight on durability, multi-dosing, and re-dosing triggers —— Registrational Phase 3 trials underway interim data estimated YE 24 —— RMAT designation granted by FDA Cash runway to deliver interim Phase 3 data* —— In-house manufacturing supports Phase 3 and initial commercialization —— Experienced employees, strong product and cell therapy IP & development know-how —— Identified milestones and results throughout 2023 + Value Creation Potential Total addressable market data for the year ended 2020 is based on certain estimates of management. This information may prove to be inaccurate because of the method by which the underlying data for the estimates was obtained or because this information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties. * Approximately $506M as of 9/30/22 Why ProKidney? Contribution to Society: Stop Kidney Failure Why ProKidney? Maximize dialysis-free living Too many CKD patients require dialysis $130B U.S. Medicare spend annually on ESRD / CKD (excludes private insurance) Approximately 75 million total CKD patients in U.S. & EU Currently, no treatment options (other than transplant) exist to stop decline of kidney function REACT® Initial Clinical Success Preservation of kidney function intended to delay/prevent kidney failure/dialysis Ongoing Phase 2 program providing insight on durability, multi-dosing, and re-dosing triggers Registrational Phase 3 trials underway proact interim data estimated YE 24 RMAT designation granted by FDA Value Creation Potential Experienced board and management team Cash runway to deliver interim Phase 3 data* In-house manufacturing supports Phase 3 and initial commercialization Experienced employees, strong product and cell therapy IP & development know-how Identified milestones and results throughout 2023 + Contribution to Society: Stop Kidney Failure Total addressable market data for the year ended 2020 is based on certain estimates of management. This information may prove to be inaccurate because of the method by which the underlying data for the estimates was obtained or because this information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties. * Approximately $506M as of 9/30/22 28 ProKidney

Corporate Overview March 2023 ProKidney Corporate Overview March 2023 A step closer to potential Dialysis Prevention React®[Renal Autologous Cell Therapy]
